At a Glance

How It Works
- Budesonide is a corticosteroid that reduces swelling and irritation inside the airways of the lungs.
- It calms down immune cells and inflammatory chemicals that trigger asthma symptoms like wheezing and shortness of breath.
- With regular use, the airways become less sensitive, leading to fewer flare-ups and better breathing over time.
Treatment and Efficacy
Approved indications: Inhaled budesonide is approved for long-term maintenance treatment of asthma and for prevention of asthma symptoms in adults and children, helping control chronic inflammation in the airways but not treating sudden bronchospasm.
Off-label uses: Clinicians may use inhaled or nebulized budesonide off-label for conditions such as croup in children or as part of treatment for acute asthma or COPD exacerbations in certain settings, based on clinical trials and guideline experience, though these uses are not FDA-approved and practice varies.
Efficacy expectations: Many patients notice improvement in asthma symptoms within a few days, but full benefit often takes 1–2 weeks or longer of regular use, with typical outcomes including fewer daytime and nighttime symptoms, better lung function tests (such as FEV1), and reduced need for rescue inhalers and oral steroids.
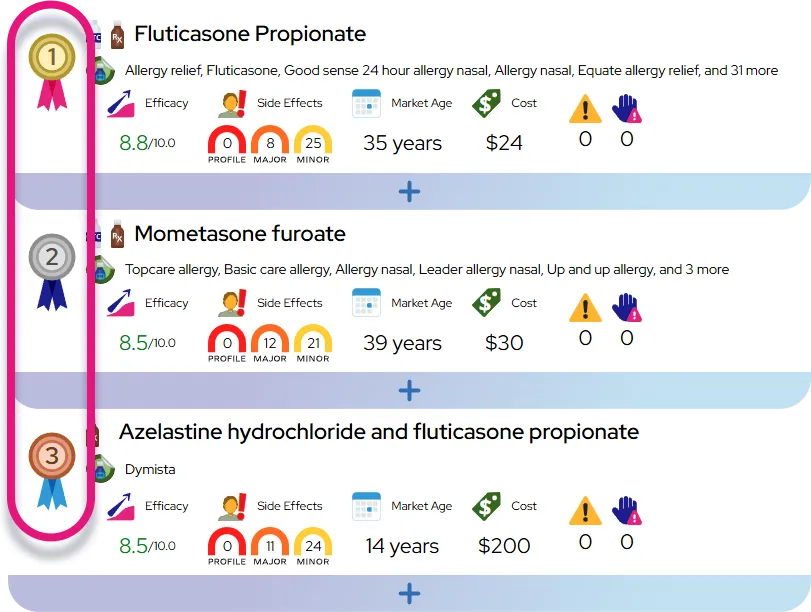
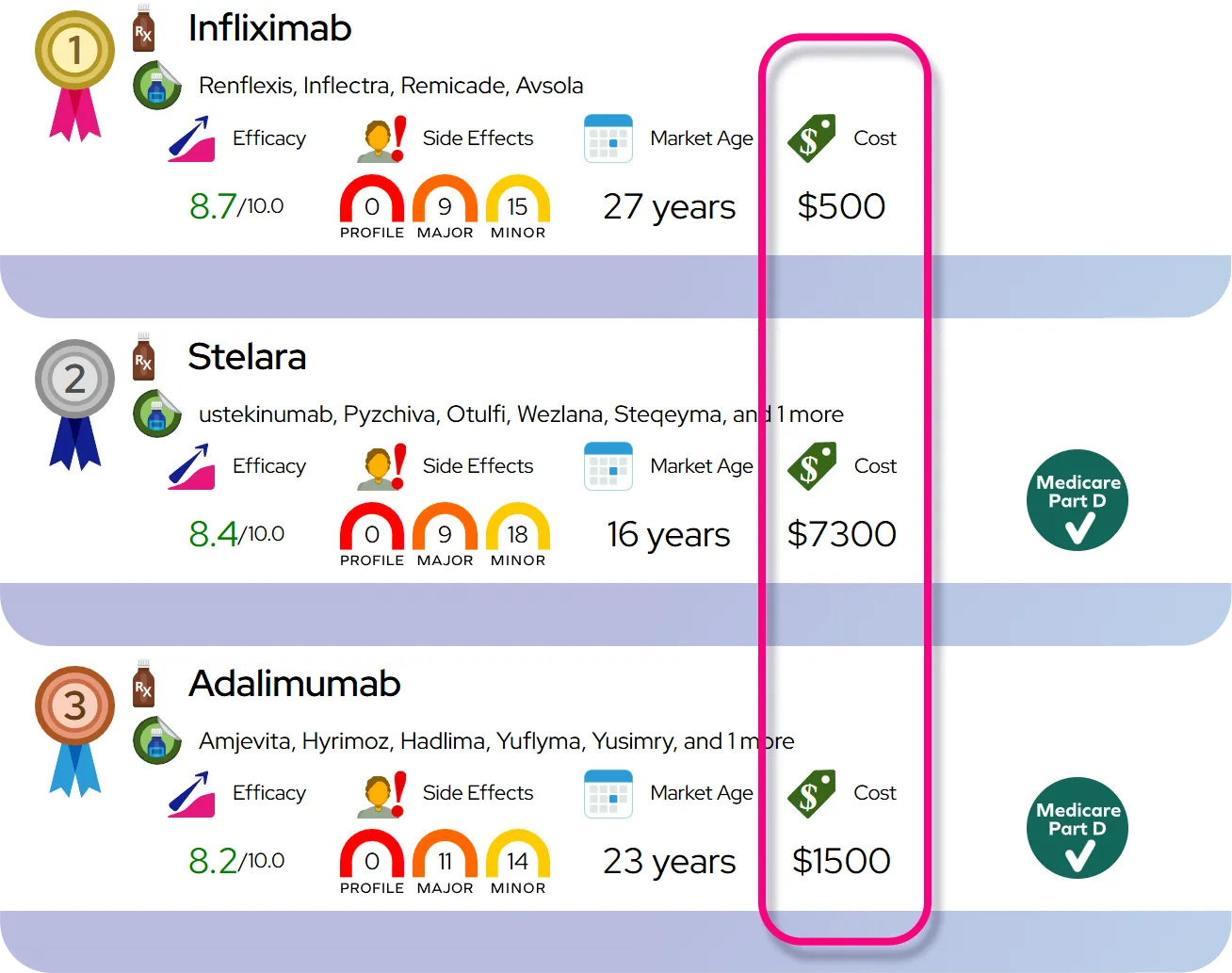
Comparison to similar drugs: At equivalent anti-inflammatory doses, budesonide provides asthma control comparable to other inhaled corticosteroids (such as fluticasone or beclomethasone), and because much of the swallowed portion is rapidly broken down by the liver, systemic steroid side effects are generally low when used at recommended doses.
Dosage and Administration
Typical dosing in adults and adolescents: For maintenance treatment of asthma with a dry-powder inhaler, many adults and adolescents start around 360 mcg twice daily (or lower in milder disease), with total daily doses usually ranging from 360–1440 mcg divided into two doses, adjusted based on asthma severity and prior steroid use.
Typical dosing in children: Children 6 years and older often use 180–360 mcg twice daily by dry-powder inhaler, while children 12 months to 8 years commonly receive 0.25–1 mg per day of nebulized budesonide (given once daily or divided twice daily) depending on symptom control and prior therapies.
How to take it: Use budesonide every day at about the same times (usually morning and evening), even when feeling well; inhale through the mouth with a forceful, deep breath for the dry-powder inhaler, or sit upright and breathe calmly through the mouthpiece or mask during nebulization until the mist stops, then rinse the mouth and spit out the water after each dose.
Special instructions: Do not use budesonide inhalation products for sudden asthma attacks—keep a rapid-acting “rescue” inhaler (like albuterol) available—and do not suddenly stop oral steroids without medical guidance when switching to inhaled budesonide, as the body may need time to recover normal adrenal function.
Missed dose guidance: If a dose is missed, take it as soon as remembered unless it is almost time for the next dose; if it is close to the next scheduled dose, skip the missed dose and resume the regular schedule, and do not double doses.
Overdose: Accidental use of a few extra inhalations is unlikely to cause serious problems in most people, but repeated high doses over time can lead to systemic steroid side effects (such as easy bruising, weight gain, or fatigue); in case of significant overdose or concern, contact a healthcare professional or poison control center right away.
Safety and Side Effects
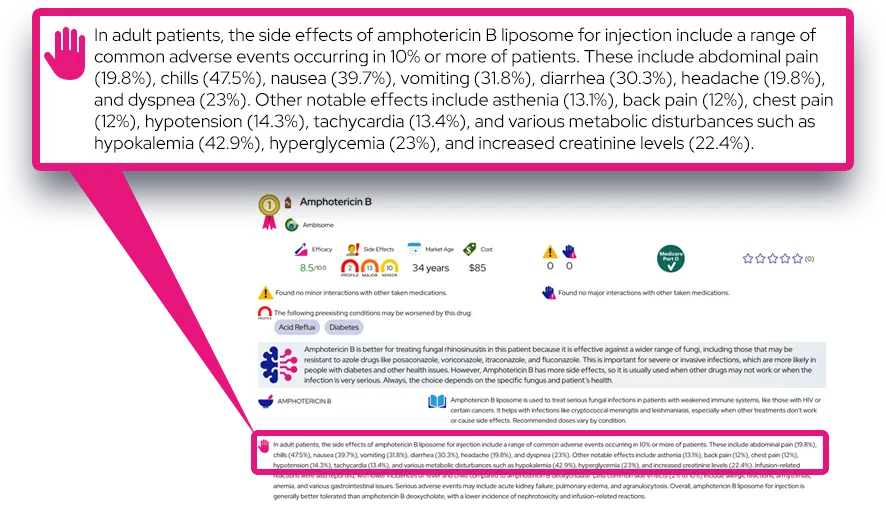
Common side effects: Frequently reported effects include hoarseness or voice changes, sore throat, cough, dry mouth or throat irritation, oral thrush (yeast infection in the mouth), and mild upper respiratory infections; these are usually mild to moderate and tend to appear within the first weeks of treatment or after dose increases.
Reducing minor side effects: Using a spacer with compatible devices, inhaling slowly and correctly, rinsing the mouth and spitting after each dose, and brushing the teeth at bedtime can lower the risk of hoarseness and oral thrush.
Serious or rare adverse effects: Though uncommon at inhaled doses, long-term high-dose use or use with strong drug interactions can cause adrenal suppression, slowed growth in children (small average reduction in growth velocity), decreased bone mineral density, eye problems (cataracts or glaucoma), and increased susceptibility to infections; sudden worsening of breathing right after a dose (paradoxical bronchospasm), severe allergic reactions (rash, swelling, trouble breathing), or signs of systemic steroid effects (weight gain, facial rounding, fatigue, weakness) require urgent medical attention.
Warnings and precautions: Inhaled budesonide should not be used as a rescue inhaler for acute asthma attacks; use caution in people with active or latent infections (such as tuberculosis, untreated fungal, bacterial, or viral infections), certain eye diseases (glaucoma, cataracts), osteoporosis, or liver disease, and in children long-term growth should be monitored.
Pregnancy and breastfeeding: Inhaled budesonide has substantial safety experience in pregnancy and is often considered a preferred inhaled steroid when an inhaled corticosteroid is needed; only small amounts pass into breast milk with usual doses, and it is generally considered compatible with breastfeeding, but treatment decisions should be individualized.
Comparative safety: Overall, budesonide has a safety profile similar to other inhaled corticosteroids, with low systemic exposure at recommended doses and a favorable benefit–risk balance when used regularly for persistent asthma.
Side-effect reporting and safety updates: Patients can report suspected side effects to their clinician and directly to the FDA through the MedWatch program (online or by phone), and updated safety communications are posted on the FDA’s public website and sometimes in medication guides provided with the product.
Interactions and Precautions
Drug interactions: Strong inhibitors of the enzyme CYP3A4—such as certain antifungals (ketoconazole, itraconazole), some HIV or hepatitis C medicines (ritonavir and other protease inhibitors), and some macrolide antibiotics (like clarithromycin)—can increase blood levels of budesonide and raise the risk of systemic steroid effects; clinicians may adjust the dose, choose an alternative inhaled steroid, or avoid combinations when possible.
Other medicines and products: Using budesonide with other steroids (oral, injected, or nasal) or with potent immunosuppressants can add to the risk of adrenal suppression and infection; while interactions with most over-the-counter medicines and supplements are limited, patients should mention chronic use of herbal products (such as high-dose St John’s wort, which induces CYP3A4) and avoid starting or stopping interacting drugs without medical advice.
Food, alcohol, and lifestyle: Grapefruit and grapefruit juice can increase levels of some CYP3A4-metabolized drugs and may modestly increase systemic exposure to budesonide, though this is less pronounced with inhaled therapy; moderate alcohol use has no direct interaction but heavy alcohol use and liver disease can affect steroid metabolism.
Precautions and contraindications: Use budesonide with caution in people with untreated infections (tuberculosis, certain fungal, bacterial, or viral infections), severe liver impairment, eye diseases such as glaucoma or cataracts, or significant bone loss, and in those transitioning from long-term oral steroids who may be at higher risk for adrenal insufficiency.
Monitoring needs: Clinicians may periodically check asthma control, rescue-inhaler use, and lung function (spirometry), monitor children’s growth, assess for signs of adrenal suppression or systemic steroid effects in high-risk patients, and arrange periodic eye exams and bone density testing in those on long-term high-dose inhaled steroids or with additional risk factors.
Common Questions and Answers
Q: Is budesonide a rescue inhaler or a controller medicine?
A: Budesonide is a controller (maintenance) inhaled steroid that works over time to reduce airway inflammation and prevent asthma symptoms, so you still need a separate fast-acting rescue inhaler for sudden breathing problems.
Q: How long does it take for inhaled budesonide to start working?
A: Some people notice easier breathing and fewer symptoms within a few days, but the full benefit usually appears after 1–2 weeks or more of taking it every day as prescribed.
Q: Do I really need to rinse my mouth after using budesonide?
A: Yes, rinsing your mouth with water and spitting it out after each dose helps reduce the risk of oral thrush and hoarseness while still keeping the medicine working in your lungs.
Q: Can budesonide stunt my child’s growth?
A: Inhaled budesonide and other inhaled steroids can slightly slow growth in some children, especially at higher doses, but the average effect is small, and good asthma control is important for overall health; clinicians usually use the lowest effective dose and monitor growth over time.
Q: Is budesonide safe to use during pregnancy?
A: Inhaled budesonide has extensive pregnancy safety data and is often considered a preferred inhaled corticosteroid for pregnant patients who need one, but treatment decisions should always be individualized with the obstetric and asthma care teams.
Q: What should I do if I keep needing my rescue inhaler while on budesonide?
A: Frequent rescue-inhaler use (for example, more than two days per week for asthma symptoms) suggests your asthma may not be well controlled, so you should contact your healthcare provider to review inhaler technique, adherence, triggers, and whether your budesonide dose or treatment plan needs adjustment.
Better Treatment, Lower Cost – No Catch.
Find safer, more effective medications with fewer side effects – often for less money. It’s fast, free, and personalized. Learn More →
Disposal Guidance
Storage: Keep inhaled budesonide at room temperature (generally 68–77°F / 20–25°C), away from excess heat, moisture, and direct sunlight; store inhalers tightly closed and dry, and keep nebulizer ampules (Respules) in their foil pouch or carton until use.
Handling: Do not wash the dry-powder inhaler with water; if needed, gently wipe the mouthpiece with a dry tissue or cloth, and use nebulizer ampules only with a compatible jet nebulizer and mouthpiece or face mask as directed.
Disposal: Discard the inhaler when the dose counter reaches zero or after the time frame noted on the package, and throw away any opened or outdated nebulizer ampules; place used devices and ampules in household trash unless your pharmacist or local waste program provides specific inhaler recycling or take-back options, and do not flush medicines down the toilet unless specifically instructed.