At a Glance

How It Works
Fluticasone propionate is a corticosteroid that works mainly on the lining of the nose to reduce inflammation.- It blocks the release of inflammatory chemicals (such as histamine and cytokines) that cause swelling, itching, and mucus.
- With regular daily use, the nasal passages become less swollen, making it easier to breathe and reducing sneezing and runny nose.
- Because very little is absorbed into the bloodstream at recommended doses, its effects are mostly local in the nose.
Treatment and Efficacy
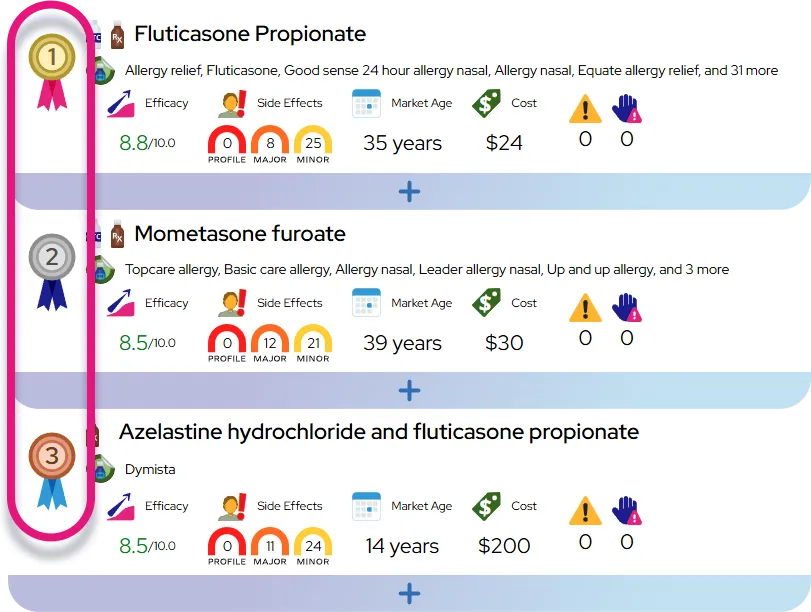
Approved indications: Intranasal fluticasone propionate sprays are FDA‑approved for management of nasal symptoms of seasonal and perennial allergic rhinitis and perennial nonallergic rhinitis in adults and children 4 years and older, and exhalation‑delivery formulations (such as XHANCE) are approved for chronic rhinosinusitis with or without nasal polyps in adults.
Off‑label uses (evidence level): Clinicians may also use fluticasone nasal sprays off‑label as part of treatment for acute or chronic rhinosinusitis in children, adenoid hypertrophy, or otitis media with effusion; support comes from small clinical trials and guideline‑based expert opinion, but these uses are not formally FDA‑approved.
Efficacy expectations:
- Some people notice improvement in congestion, sneezing, and runny nose within 12–24 hours, but maximum benefit usually takes several days to 1–2 weeks of consistent daily use.
- In clinical studies, fluticasone nasal sprays significantly reduce total nasal symptom scores and improve daily functioning and sleep compared with placebo when used regularly.
- Compared with oral antihistamines, intranasal corticosteroids like fluticasone generally provide superior relief of nasal congestion and overall allergic rhinitis symptoms and offer similar overall efficacy to other intranasal steroids when used at equivalent anti‑inflammatory doses.
Dosage and Administration
Typical dosing (adults and adolescents): For allergic or nonallergic rhinitis, a common starting dose of fluticasone propionate 50 mcg nasal spray is 2 sprays in each nostril once daily (total 200 mcg/day), with many patients stepping down to 1 spray per nostril once daily once symptoms are controlled; some adults may instead use 1 spray per nostril twice daily (still 200 mcg/day).
Typical dosing (children ≥4 years): A usual dose is 1 spray (50 mcg) in each nostril once daily (total 100 mcg/day), and some products allow an increase to 2 sprays per nostril once daily (200 mcg/day) if symptoms are not adequately controlled, always using the lowest effective dose.
Chronic rhinosinusitis with or without nasal polyps (adult exhalation‑delivery formulations): Products such as XHANCE are generally given as 1 spray per nostril twice daily, with some patients using 2 sprays per nostril twice daily; patients should follow the specific device’s instructions.
How to use: Gently blow the nose, shake the bottle, prime the pump if new or not used for a while, then insert the tip into the nostril while slightly tilting the head forward, aim away from the center wall of the nose, and sniff gently while spraying; repeat for the other nostril and wipe the tip and replace the cap.
Special instructions: Use the spray at the same time each day, do not exceed the maximum recommended sprays per day, and clean the nozzle regularly according to the package insert to prevent clogging.
Missed dose: If you miss a dose, use it when you remember on the same day, but if it is almost time for the next scheduled dose, skip the missed dose and resume the regular schedule without doubling up.
Overdose: Accidental use of a few extra sprays is unlikely to cause serious problems, but high doses for long periods may increase the risk of systemic steroid effects and adrenal suppression; if a large amount is used or swallowed, or if concerning symptoms develop, contact a healthcare professional or poison control center.
Safety and Side Effects
Common side effects:
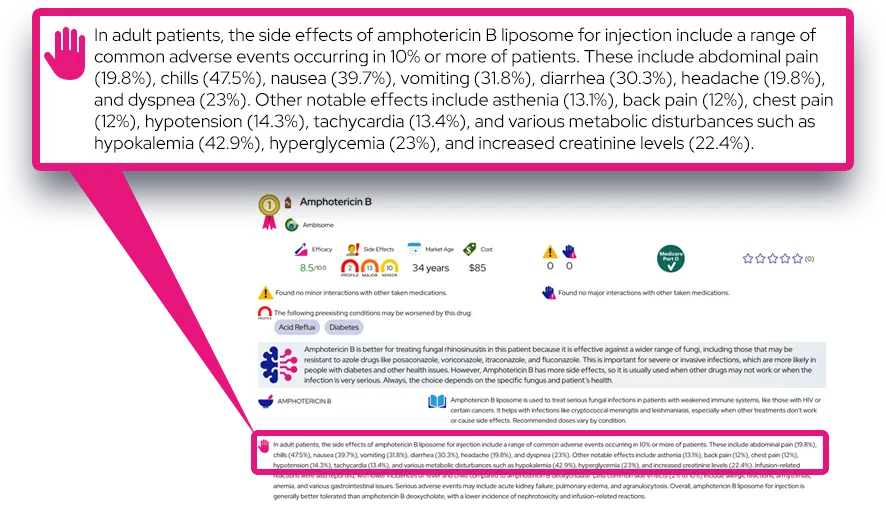
- Nasal irritation, burning or stinging, sneezing, headache, cough, dry or sore throat, or an unpleasant taste or smell are relatively common and are usually mild and temporary.
- Nosebleeds (epistaxis) can occur, especially with higher doses or if the spray is aimed at the nasal septum; adjusting technique and using the lowest effective dose can reduce this risk.
- Most common side effects appear within the first days to weeks of treatment and often lessen as the nose adapts to the spray.
Serious or rare adverse effects (seek care immediately):
- Severe or persistent nosebleeds, painful sores or ulcers inside the nose, or signs of a hole in the nasal septum (whistling sound when breathing).
- Signs of severe allergy such as rash, hives, swelling of the face, lips, tongue, or throat, trouble breathing, or sudden dizziness.
- White patches in the nose or throat suggesting fungal infection, vision changes, eye pain, or very unusual tiredness, weight gain in the face/upper body, or easy bruising that might indicate systemic steroid effects.
Warnings and precautions:
- Use with caution and medical supervision if you have recent nasal surgery, trauma, or ulcers; treatment is usually delayed until healing has occurred.
- Children using intranasal steroids for long periods should have their growth monitored, and the lowest effective dose should be used.
- People with glaucoma, cataracts, significant liver disease, active or recurrent infections (especially tuberculosis, untreated fungal, or herpes simplex eye infections) should discuss risks and monitoring with their clinician.
- In pregnancy and breastfeeding, intranasal fluticasone is often used when needed because systemic absorption is low, but it should be used at the minimum effective dose after a risk‑benefit discussion with the prescriber.
Comparative safety: At recommended intranasal doses, fluticasone propionate has a low rate of systemic steroid side effects and a safety profile comparable to other modern intranasal corticosteroids; most problems are local to the nose and related to technique or prolonged use.
Reporting and safety updates: Side effects can be reported to a healthcare professional and to the FDA MedWatch program (by phone or online), and up‑to‑date safety communications can be found on the FDA’s drug safety web pages.
Interactions and Precautions
Drug and supplement interactions: Strong inhibitors of the enzyme CYP3A4 (such as ritonavir, cobicistat‑containing regimens, ketoconazole, itraconazole, or clarithromycin) can increase fluticasone levels and the risk of systemic steroid effects, so combined use should be avoided or closely monitored; using multiple steroid medicines (inhaled, oral, topical) together can have additive systemic effects.
Food, alcohol, and procedures: Because this medicine is used in the nose, food does not affect its action, and there are no specific alcohol or imaging‑procedure restrictions, though heavy alcohol use may worsen nosebleeds and should be discussed with a clinician.
Conditions requiring extra caution: Tell your prescriber if you have uncontrolled infections (including tuberculosis), frequent nosebleeds, recent nasal surgery or trauma, glaucoma, cataracts, significant liver impairment, or a history of steroid‑related side effects, as dose adjustments or extra monitoring may be needed.
Monitoring needs: For long‑term or high‑dose use, clinicians may periodically check nasal mucosa for irritation or fungal infection, monitor eye pressure or lens changes in people at risk for glaucoma or cataracts, and monitor growth in children and signs of adrenal suppression in anyone on prolonged higher‑dose therapy, especially if also taking strong CYP3A4 inhibitors.
Common Questions and Answers
Q: How long does it take for fluticasone propionate nasal spray to start working?
A: Some people notice relief of congestion and sneezing within the first day, but it often takes several days to a week of daily use to feel the full benefit, so it should be used regularly even if symptoms are not immediately gone.
Q: Can I use fluticasone nasal spray every day for a long time?
A: Many people use it daily during allergy seasons or year‑round under medical supervision; the goal is to use the lowest dose that keeps symptoms controlled, and children or long‑term users may need periodic checks for growth and eye health.
Q: Is fluticasone nasal spray the same as an oral steroid?
A: No, the nasal spray is designed to act mainly on the nasal lining with very low amounts reaching the bloodstream at recommended doses, so it has far fewer whole‑body steroid effects than typical oral steroid courses.
Q: Can I use fluticasone nasal spray with my antihistamine or asthma inhaler?
A: Fluticasone nasal spray is often combined with oral or intranasal antihistamines and with asthma inhalers, but you should tell your clinician about all steroid‑containing medications so that your total steroid exposure can be considered.
Q: What should I do if my nose keeps bleeding while using the spray?
A: Stop the spray temporarily, avoid blowing or picking the nose, aim the nozzle away from the center of the nose when you restart, and contact your healthcare professional if bleeding is frequent, heavy, or does not improve.
Better Treatment, Lower Cost – No Catch.
Find safer, more effective medications with fewer side effects – often for less money. It’s fast, free, and personalized. Learn More →
Disposal Guidance
Storage: Keep the nasal spray at room temperature (about 68°F to 77°F / 20°C to 25°C), protected from freezing and excessive heat; store it upright with the cap on, and keep it out of reach of children and pets.
Use period: Check the bottle or package for the number of sprays or months it can be used after first opening, and discard any remaining medication after that time or after the labeled expiration date, whichever comes first.
Disposal: Do not burn or puncture the bottle; when empty or expired, discard it in household trash out of reach of children and animals, or follow any local pharmacy or community drug take‑back program instructions.