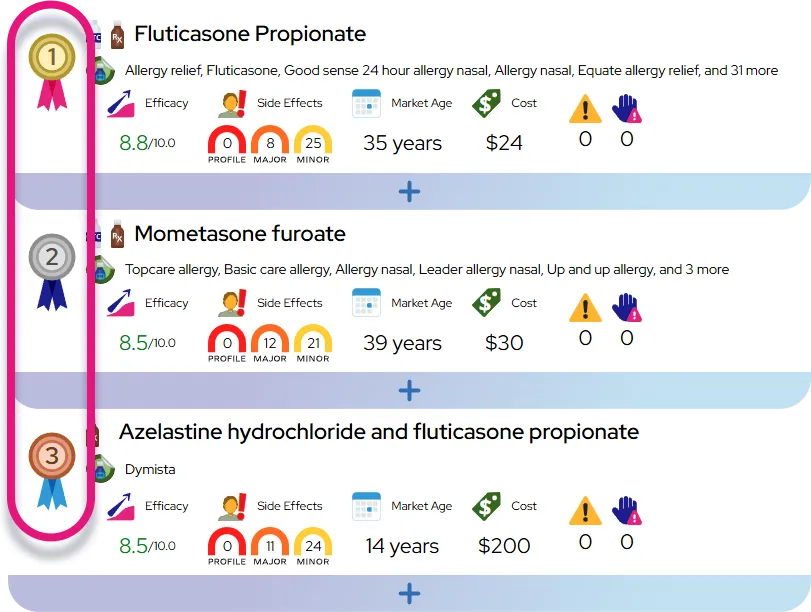
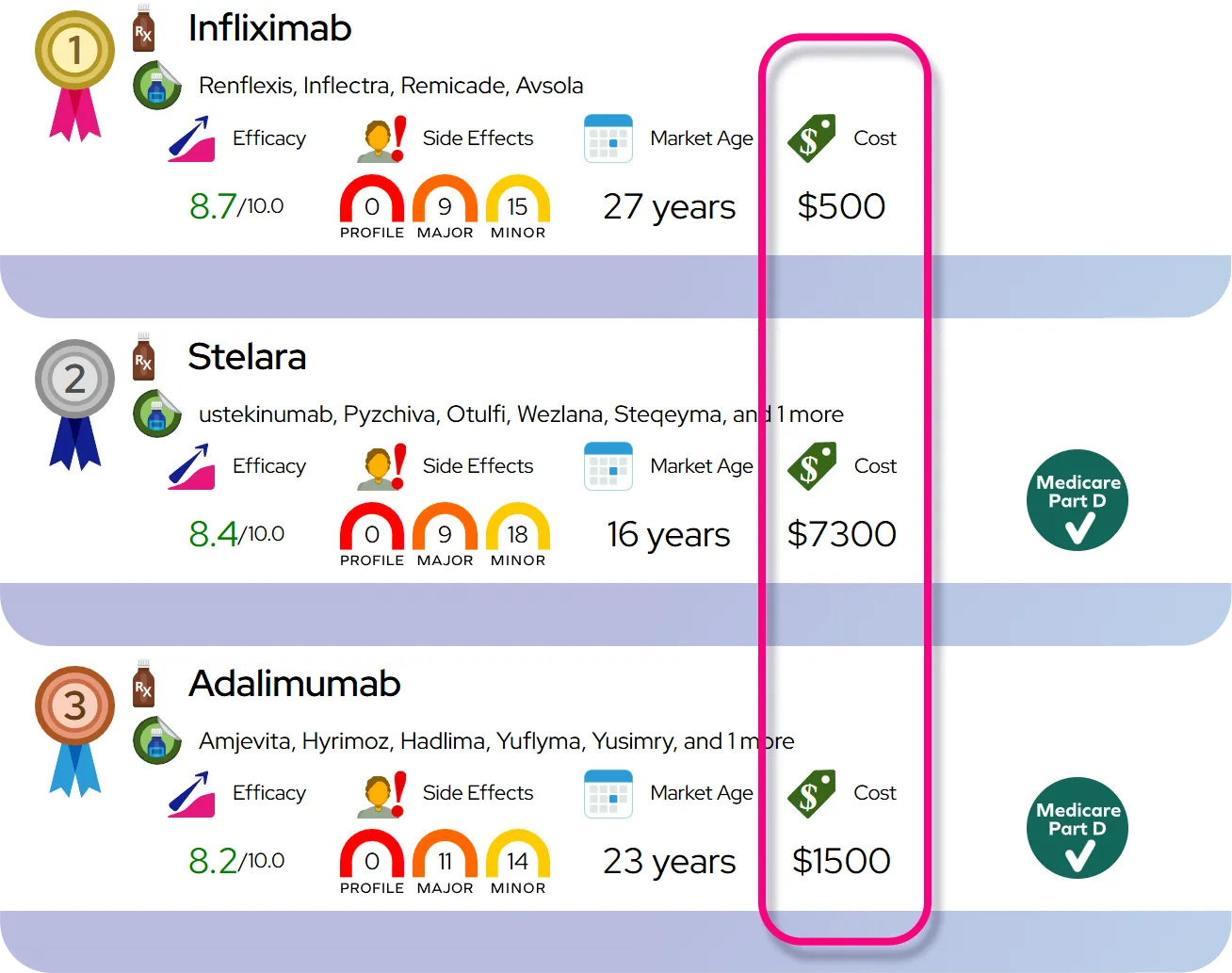
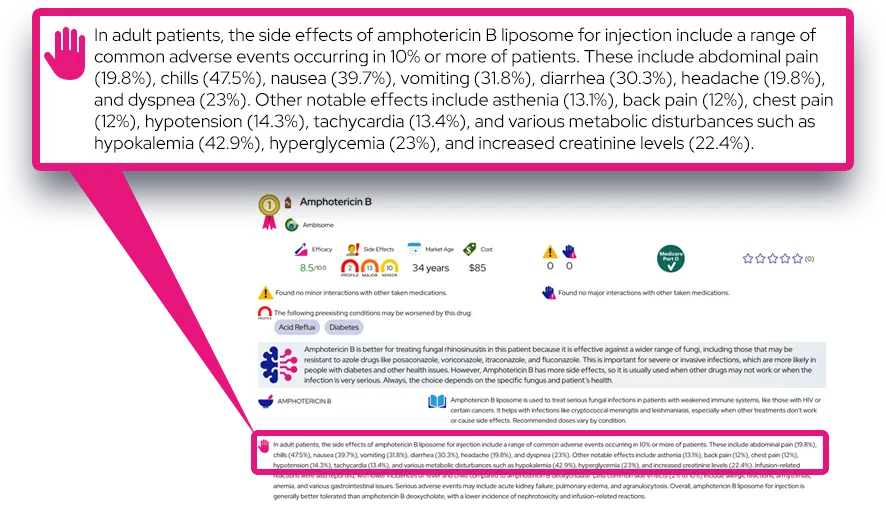
At a Glance

How It Works
Inhaled formoterol fumarate is a long-acting beta2-agonist (LABA) that relaxes muscles in the airways so breathing becomes easier.- It attaches to beta2 receptors in the bronchial muscles, causing them to relax and the airways to widen (bronchodilation).
- It starts to work within a few minutes and usually lasts about 12 hours, helping to prevent symptoms such as wheeze, chest tightness, and shortness of breath.
- It is used regularly as a controller medicine and is not meant to replace a fast-acting rescue inhaler for sudden breathing problems unless specifically directed in a combination regimen.
Treatment and Efficacy
Approved indications: Inhaled formoterol fumarate is approved as a long-acting bronchodilator for maintenance treatment of asthma only when used in fixed-dose combination with an inhaled corticosteroid, for maintenance treatment of chronic obstructive pulmonary disease (COPD, including chronic bronchitis and emphysema) in adults, and in some formulations for prevention of exercise-induced bronchospasm.
Off-label uses and evidence: Clinicians may use formoterol-containing regimens off label as both maintenance and reliever therapy in asthma (a strategy often called single-inhaler maintenance and reliever therapy), supported by moderate to high-quality evidence and international guideline recommendations, though specific product labels and national approvals vary.
Efficacy expectations: Most people notice easier breathing within minutes of a dose, with peak effect in about 1–3 hours and bronchodilation that typically lasts around 12 hours; over weeks to months, regular use in appropriate regimens improves lung function, reduces day- and nighttime symptoms, and lowers the risk of exacerbations, with overall benefit comparable to other LABAs and similar or better speed of onset compared with some alternatives.
Dosage and Administration
Typical dosing: For asthma or COPD maintenance, usual inhaled doses are 1–2 puffs (often totaling about 4.5–12 micrograms of formoterol) twice daily via a metered-dose or dry-powder inhaler, or about 20 micrograms via nebulizer solution twice daily in adults with COPD, with exact dose and device determined by the specific product and patient age.
How to take it: Inhale the medicine by mouth using the prescribed device with proper technique, usually at the same times each morning and evening; do not swallow the capsules or solution, and use formoterol only as part of the regimen your clinician has prescribed, typically together with an inhaled corticosteroid for asthma.
Special dosing instructions: Do not use extra doses for sudden breathing problems unless your plan specifically uses a formoterol combination inhaler as a reliever; for products approved to prevent exercise-induced bronchospasm, the dose is usually taken about 15 minutes before activity, not repeated more often than recommended, and the total daily dose must remain within the labeled maximum.
Missed dose: If a dose is missed, take it when remembered unless it is almost time for the next scheduled dose, in which case skip the missed dose and resume the regular schedule without doubling.
Overdose: Taking more than prescribed can lead to severe tremor, chest pain, very fast or irregular heartbeat, high or low blood pressure, nervousness, headache, or seizures; in case of suspected overdose, seek emergency medical care or contact a poison control center immediately.
Safety and Side Effects
Common side effects: These may include headache, mild tremor or nervousness, palpitations or a sense of fast heartbeat, cough, throat irritation, and upper respiratory infections; they are usually mild to moderate, often appear soon after starting treatment or increasing the dose, and may lessen as the body adjusts.
Serious or rare adverse effects: Seek urgent medical attention for chest pain, severe or worsening shortness of breath (including paradoxical bronchospasm right after inhalation), very fast or irregular heartbeat, severe dizziness or fainting, seizures, signs of allergic reaction (rash, swelling of face or throat, trouble breathing), or symptoms of low potassium such as muscle weakness or severe cramps.
Warnings and precautions: Formoterol must not be used alone for asthma and should always be combined with an inhaled corticosteroid to reduce the risk of asthma-related hospitalization and death; use with caution in people with heart disease, high blood pressure, arrhythmias, hyperthyroidism, diabetes, seizure disorders, or those prone to low potassium, and in children only within approved age ranges for the specific product.
Pregnancy, breastfeeding, and age: Experience in pregnancy and breastfeeding is limited, so the drug is generally used when the expected benefit in controlling asthma or COPD outweighs potential risks; dosing and approved use depend on age, with certain strengths and devices restricted to older children or adults.
Comparative safety: The side-effect profile of formoterol is broadly similar to other LABAs, with cardiovascular and metabolic effects mainly at higher doses or in susceptible patients; when used as directed with an inhaled corticosteroid, the overall safety record is favorable for long-term control of asthma and COPD.
Safety information and reporting: Patients and caregivers should report troublesome or unexpected side effects to their prescriber and can also report them to national pharmacovigilance programs (such as FDA MedWatch in the United States) and consult regulatory agency websites for updated safety alerts and communication on formoterol-containing inhalers.
Interactions and Precautions
Drug interactions: Formoterol’s effects may be increased by other sympathomimetic drugs (such as some decongestants), monoamine oxidase inhibitors, and tricyclic antidepressants; it can have additive heart and blood pressure effects with other stimulants, and its heart rhythm effects may be worsened when combined with medicines that prolong the QT interval or with high doses of diuretics, xanthines, or systemic steroids that lower potassium.
Other medicines and substances: Nonselective beta-blockers (such as propranolol) can blunt or reverse the bronchodilator effect, while cardioselective beta-blockers may still interfere and are used cautiously; large amounts of caffeine or herbal stimulants (such as ephedra) can increase jitteriness or palpitations when used with formoterol, and alcohol may heighten dizziness or perceived heartbeat changes in sensitive individuals.
Medical conditions requiring caution: Use with particular care in people with coronary artery disease, heart failure, arrhythmias, hypertension, hyperthyroidism, diabetes, seizure disorders, or a history of severe hypokalemia, and in those who require frequent rescue inhaler use or have unstable asthma or COPD, as they may need closer monitoring or adjustment of therapy.
Monitoring: Clinicians may monitor lung function, symptom control, rescue-inhaler use, heart rate and blood pressure, and, in higher-risk patients or those on interacting medicines, serum potassium and sometimes an electrocardiogram (ECG) to watch for rhythm changes.
Procedures and anesthesia: Inform surgeons, anesthesiologists, and other healthcare professionals that you use a formoterol-containing inhaler before surgery or procedures that involve anesthesia, as they may need to consider its cardiovascular and respiratory effects when planning care.
Common Questions and Answers
Q: Is formoterol a controller inhaler or a rescue inhaler?
A: Formoterol is primarily a controller medicine used regularly to keep asthma or COPD under control, and a separate fast-acting rescue inhaler is usually still needed unless your clinician has placed you on a specific formoterol-containing regimen that also covers relief.
Q: How long does it take for formoterol to start working and how long does it last?
A: Most people feel some relief within a few minutes of inhalation, with effects that typically last about 12 hours when taken at the prescribed dose.
Q: Can I use formoterol by itself for asthma?
A: No, formoterol must not be used alone for asthma and should always be paired with an inhaled corticosteroid, usually in a fixed combination inhaler, to reduce the risk of severe asthma attacks and asthma-related death.
Q: What should I do if my breathing suddenly gets worse after using formoterol?
A: Use your quick-relief inhaler if prescribed, do not take extra doses of formoterol unless instructed, and seek urgent medical attention because sudden worsening after a dose may indicate paradoxical bronchospasm or poorly controlled disease.
Q: Do I still need an inhaled steroid if I feel well on formoterol?
A: Yes, people with asthma generally need to continue their inhaled corticosteroid as directed even when they feel well, because the steroid treats airway inflammation while formoterol mainly relaxes the airway muscles.
Q: Can I stop formoterol suddenly if my symptoms improve?
A: Do not stop or change your formoterol-containing inhaler on your own; talk with your healthcare provider, who can decide whether and how to step down treatment safely based on your asthma or COPD control.
Better Treatment, Lower Cost – No Catch.
Find safer, more effective medications with fewer side effects – often for less money. It’s fast, free, and personalized. Learn More →
Disposal Guidance
Storage: Keep inhalers or nebulizer vials at room temperature, away from excessive heat, open flames, and freezing; store in a dry place with the mouthpiece cap on or foil pouch closed, and do not use the medicine past the expiration date or beyond the labeled number of actuations.
Handling: Do not puncture, break, or burn pressurized canisters, even when they appear empty, and keep all inhalation devices out of reach of children and pets.
Disposal: Follow the instructions in the patient leaflet or from your pharmacy for discarding used or expired inhalers and nebulizer vials; if no specific instructions are provided, return them to a medicine take-back program or ask your pharmacist how to dispose of them with household trash without crushing or burning the containers.