At a Glance
How It Works
Rhapsido is a targeted Bruton's tyrosine kinase (BTK) inhibitor that calms an overactive immune response in chronic spontaneous urticaria.- Blocks BTK inside immune cells: It binds to BTK in mast cells and basophils, turning down the signals that trigger hives and swelling.
- Reduces histamine release: By calming these cells, it lowers the release of histamine and other inflammatory chemicals into the skin.
- Lowers hives and itch: With less histamine, most people have fewer hives, less itching, and fewer flares over time.
Treatment and Efficacy
Approved indications: Rhapsido is FDA-approved for chronic spontaneous urticaria (CSU) in adults who remain symptomatic despite H1 antihistamine treatment and is not indicated for other types of hives.
Off-label and investigational uses: There are no well-established off-label uses in routine practice; remibrutinib is being studied in conditions such as chronic inducible urticaria, hidradenitis suppurativa, and food allergy, but these uses are investigational and should generally be limited to clinical trials rather than standard prescribing.
Efficacy expectations: In phase 3 REMIX trials, many patients had meaningful reductions in itch and hive scores by about week 2, and by week 12 roughly one-third achieved complete clearance of hives, with more achieving substantial but not complete improvement; however, some patients respond only partially or not at all. Compared with placebo, Rhapsido produced significantly better control of CSU symptoms. There are no head-to-head trials versus biologics such as omalizumab or dupilumab, but Rhapsido offers a convenient oral, twice-daily alternative to injectable advanced therapies, with efficacy in the range expected for a modern targeted CSU treatment.
Dosage and Administration
Typical dosing: The recommended adult dose is 25 mg taken orally twice daily, usually one tablet in the morning and one at night, with or without food.
How to take it: Swallow the tablets whole with water; do not split, crush, or chew them. Try to take doses at about the same times each day, and continue your background H1 antihistamine as directed unless your clinician advises otherwise.
Special dosing instructions: Because Rhapsido increases bleeding risk, treatment should be interrupted for 3 to 7 days before and after surgery or invasive procedures, depending on the procedure and your bleeding risk, and coordinated with your surgeon and prescribing clinician. Use is not recommended in patients with hepatic impairment, and clinicians will review other medicines to avoid strong or moderate CYP3A4 inhibitors or inducers that could significantly change Rhapsido levels.
Missed doses and overdose: If you miss a dose, skip it and take your next scheduled dose at the usual time; do not take extra tablets to make up for a missed dose. In case of suspected overdose, contact poison control or emergency services immediately, as there is no specific antidote and treatment focuses on monitoring and managing bleeding or other symptoms.
Safety and Side Effects
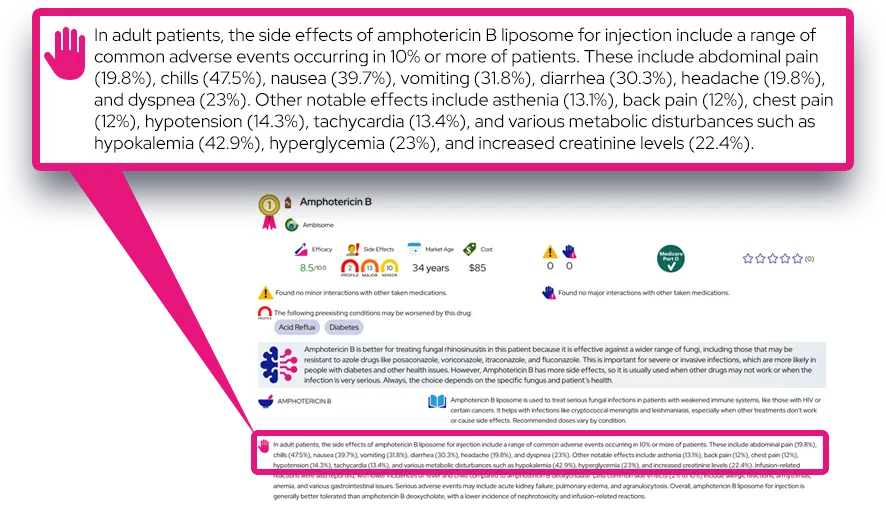
Common side effects: The most common side effects (seen in at least a few percent of patients) include nasopharyngitis or cold-like symptoms, various mild bleeding events such as easy bruising or nosebleeds, headache, nausea, and abdominal pain; these are usually mild to moderate and often start within the first weeks of treatment.
Serious or rare adverse effects: Rhapsido increases the risk of mucocutaneous bleeding, and more serious bleeding (such as heavy gastrointestinal bleeding, coughing or vomiting blood, black or bloody stools, or signs of brain bleeding like severe headache, confusion, or sudden weakness) needs urgent medical attention and usually stopping the drug. Rare liver test abnormalities and serious infections have been observed but occurred at low rates similar to placebo in trials; any signs of jaundice, dark urine, persistent fever, or severe fatigue should prompt immediate evaluation.
Warnings and precautions: Rhapsido should be avoided in patients with any degree of hepatic impairment because drug levels rise substantially in liver disease. It is not approved for children, and safety and effectiveness in people under 18 years are unknown. Use caution in people with current or past bleeding problems or those taking blood thinners or other drugs that affect clotting, and interrupt treatment 3 to 7 days before and after planned surgery or invasive procedures. Live or live-attenuated vaccines should be avoided during treatment, and effects in pregnancy and breastfeeding are unknown, so treatment decisions require individualized risk–benefit discussion.
Overall safety profile: In clinical studies for CSU, Rhapsido had a generally favorable and predictable safety profile without the need for routine laboratory monitoring, though careful clinical monitoring for bleeding is important; its safety compares reasonably well with other targeted CSU therapies, with bleeding risk being the main distinguishing concern.
Reporting side effects and safety updates: Patients can report suspected side effects to the FDA MedWatch program or to Novartis (the manufacturer) using the contact information in the Patient Information leaflet, and clinicians should consult current prescribing information and regulatory updates for the latest safety communications.
Interactions and Precautions
Prescription and OTC drug interactions: Rhapsido levels can rise markedly with strong or moderate CYP3A4 inhibitors (such as certain antivirals and antifungals, and some macrolide antibiotics) and fall with strong or moderate CYP3A4 inducers (such as rifampin, carbamazepine, phenytoin, and some seizure medicines), so these combinations are generally avoided. Because Rhapsido increases bleeding risk, combining it with anticoagulants, antiplatelet drugs, or other agents that affect clotting (including some NSAIDs) can further raise bleeding risk and requires careful consideration. Rhapsido can increase blood levels of some P-glycoprotein and BCRP substrate drugs (for example digoxin or certain statins), so these may need closer monitoring for side effects.
Food, alcohol, and supplements: Rhapsido may be taken with or without food, but grapefruit and grapefruit juice should be avoided because they can increase drug levels. Moderate alcohol intake has not been specifically studied; because both alcohol and Rhapsido can contribute to bleeding or liver stress, alcohol should be limited or avoided, especially if you have liver or bleeding risks. Herbal products that affect CYP3A4 (such as St John’s wort, a CYP3A4 inducer) or clotting should only be used after review with your clinician.
Precautions and populations of concern: Rhapsido is not indicated for other forms of urticaria and should be avoided in patients with any hepatic impairment due to increased exposure. It is not approved for use in children, and safety in pregnancy and breastfeeding is unknown, so alternative options or careful specialist supervision are needed. People with current or prior significant bleeding problems, recent major surgery, or those on multiple interacting medications require individualized risk assessment before starting therapy.
Monitoring needs: Routine laboratory monitoring is not generally required, but clinicians should monitor for clinical signs of bleeding and may periodically check blood counts and liver tests based on individual risk factors. Vaccination plans should be reviewed before and during treatment, and live or live-attenuated vaccines should be avoided while taking Rhapsido.
Common Questions and Answers
Q: What is Rhapsido and who is it for?
A: Rhapsido is an oral Bruton's tyrosine kinase inhibitor used to treat chronic spontaneous urticaria (CSU) in adults who still have hives and itching despite taking H1 antihistamines; it is not approved for children or for other types of hives.
Q: How long does Rhapsido take to start working?
A: Many people notice some improvement in itch and hives within about 2 weeks, and responses continue to build over several weeks, with the full benefit often assessed around 3 months, though some individuals respond more slowly or not at all.
Q: How should I take Rhapsido tablets each day?
A: Take one 25 mg tablet by mouth twice daily, usually once in the morning and once at night, with or without food, swallowing the tablet whole with water and not splitting, crushing, or chewing it.
Q: What if I miss a dose of Rhapsido?
A: If you miss a dose, simply skip it and take your next dose at the regular time, and do not take extra tablets to make up for the missed dose.
Q: Are there foods, drinks, or medicines I should avoid while taking Rhapsido?
A: Avoid grapefruit and grapefruit juice, and let your clinician know about all prescription drugs, over-the-counter medicines, and supplements you take, especially blood thinners, strong CYP3A4 inhibitors or inducers, and drugs like digoxin or certain statins that may be affected by Rhapsido.
Q: Can I use Rhapsido if I am pregnant, planning pregnancy, or breastfeeding?
A: It is not known if Rhapsido is safe for an unborn or breastfed baby, so you should discuss pregnancy plans or breastfeeding with your clinician before starting or continuing the medicine, and you may be asked to enroll in a pregnancy registry if exposure occurs.
Better Treatment, Lower Cost – No Catch.
Find safer, more effective medications with fewer side effects – often for less money. It’s fast, free, and personalized. Learn More →
Disposal Guidance
Storage: Store Rhapsido tablets at room temperature (about 68°F to 77°F / 20°C to 25°C), with allowed short excursions between 59°F and 86°F (15°C to 30°C), and keep them in the original, tightly closed container to protect from moisture and away from children and pets.
Disposal: When no longer needed or expired, use a community medicine take-back program if possible; if none is available, mix tablets (do not crush) with an unappealing substance in a sealed container before throwing them in the household trash, and remove personal information from the prescription label; do not flush Rhapsido down the toilet or pour it down the drain unless specifically instructed.