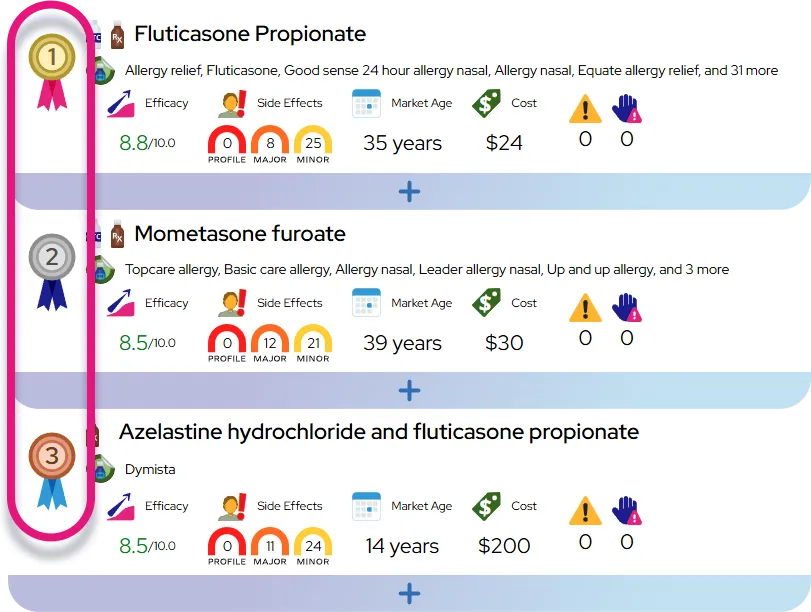
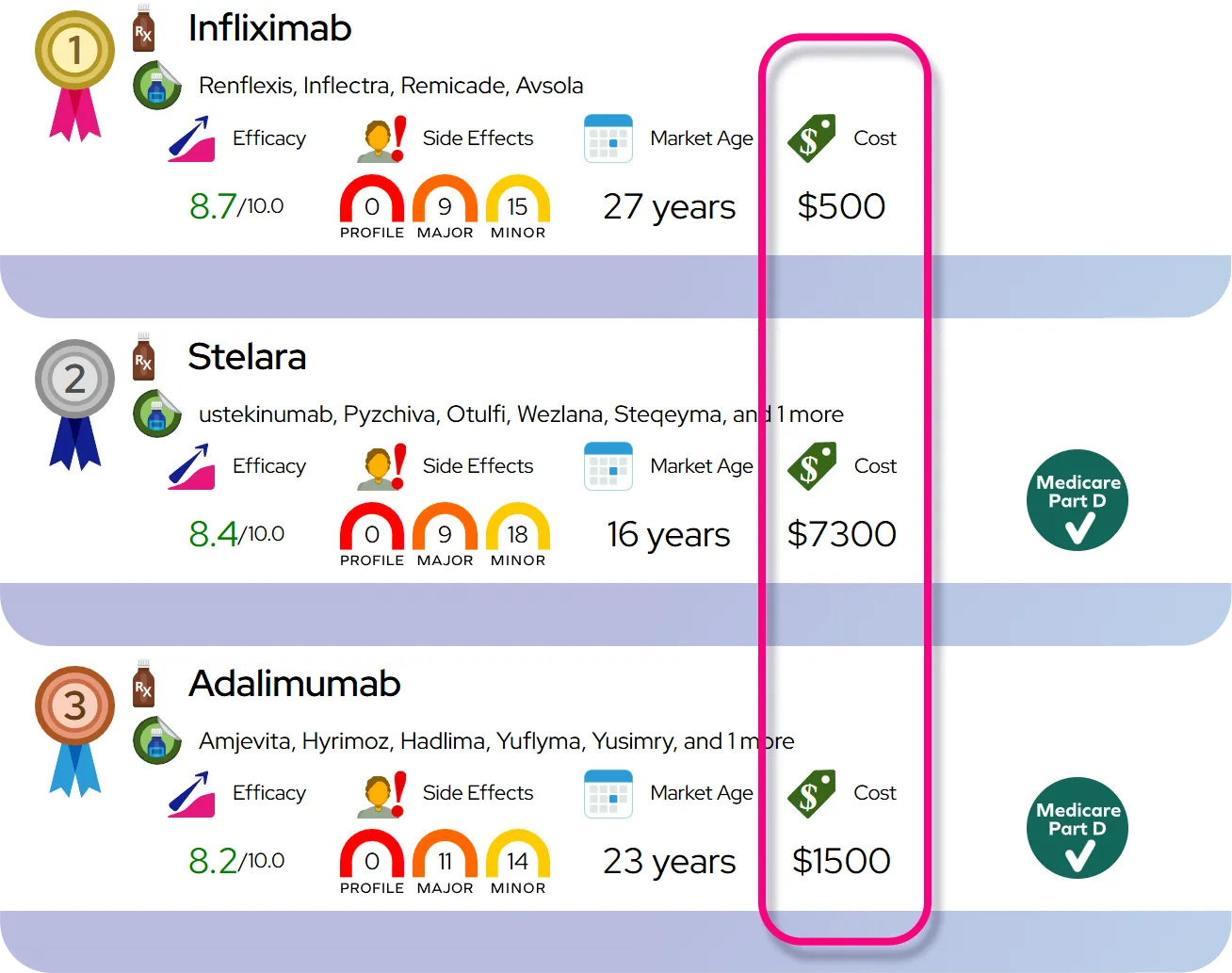
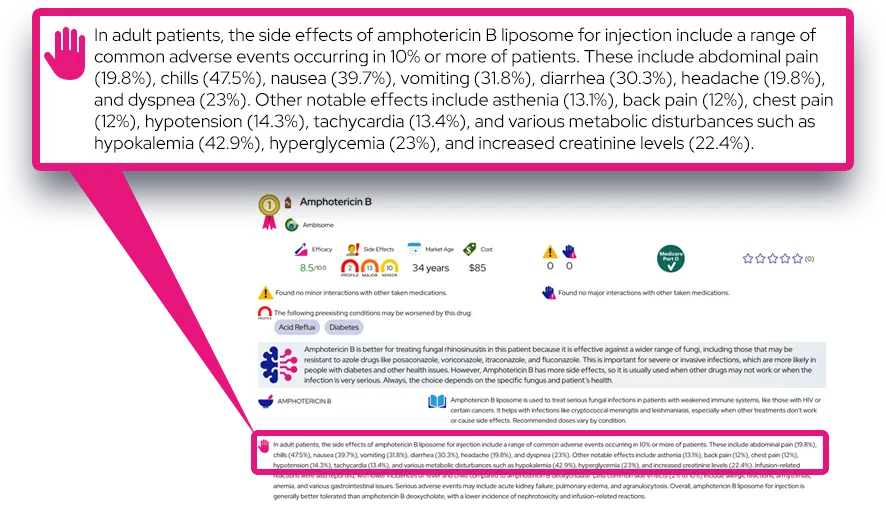
At a Glance
How It Works
- Tiotropium is a long-acting bronchodilator that relaxes muscles around the airways so they stay more open and it is easier to breathe.
- It blocks muscarinic (M3) receptors in the lungs, which normally tighten airway muscles when stimulated by the body’s acetylcholine.
- Its effects last about 24 hours, so it is taken once a day for ongoing control, not for quick relief of sudden breathing problems.
Treatment and Efficacy
Approved indications
In the U.S., inhaled tiotropium is approved for: long-term, once-daily maintenance treatment of bronchospasm and reduction of COPD exacerbations in adults with chronic obstructive pulmonary disease (chronic bronchitis and/or emphysema); and maintenance treatment of asthma in patients 6 years and older whose asthma is not adequately controlled with inhaled corticosteroids, with or without other controllers.
Off-label uses and evidence
Off-label, clinicians may consider tiotropium in some patients with more severe or difficult-to-control asthma outside labeled age ranges, or in certain overlap syndromes of asthma and COPD; evidence generally comes from randomized trials in adults and adolescents showing improved lung function and reduced exacerbations when added to standard therapy, but use should follow specialist guidance.
Efficacy expectations and time course
Improvement in lung function (such as FEV1) can often be seen within hours of the first dose, but full benefit for symptoms and exacerbation reduction is usually assessed over days to several weeks of consistent daily use; it does not replace rescue inhalers for acute attacks.
Clinical outcomes
In COPD, tiotropium typically leads to modest but clinically meaningful improvements in breathing tests, better symptom control, improved exercise tolerance, and fewer exacerbations and hospitalizations; in asthma, it can improve lung function and reduce exacerbations when added to inhaled corticosteroids with or without long-acting beta agonists.
Comparison to similar drugs
Compared with other long-acting muscarinic antagonists (LAMAs), tiotropium has a long track record of use, once-daily dosing, and similar overall efficacy; versus long-acting beta agonists, it provides bronchodilation via a different pathway and is often used in combination to optimize control.
Dosage and Administration
Typical dosing and how to take it
For COPD adults, a common regimen is 18 mcg once daily by oral inhalation of the powder contents of one capsule using the HandiHaler device, or 2.5 mcg once daily via Respimat (two inhalations of 1.25 mcg or 2.5 mcg per labeled instructions for the specific product). For asthma, typical dosing in adults and children 6 years and older using Respimat is two inhalations once daily of the labeled strength for that age group, taken at the same time each day. Capsules are for inhalation only and must not be swallowed; the inhaler should be used in an upright position with slow, deep inhalation and breath-hold as instructed.
Special dosing instructions
It is not used for sudden breathing attacks and should always be continued as maintenance therapy even when symptoms are stable unless a clinician advises otherwise. Dose adjustments for kidney or liver disease are usually not required, but extra caution is used in severe kidney impairment. Only use the inhaler device and strength prescribed, and do not double-dose or mix different tiotropium products.
Missed dose guidance
If a dose is missed, take it as soon as remembered on the same day, but if it is almost time for the next dose, skip the missed one and resume the regular schedule; do not take more than one dose in 24 hours.
Overdose
Inhalation of more than the prescribed amount may cause pronounced dry mouth, blurred vision, fast heartbeat, difficulty urinating, or severe breathing problems; in suspected overdose, seek emergency medical attention or contact a poison control center immediately, bringing the inhaler or packaging if possible.
Safety and Side Effects
Common side effects
Common side effects include dry mouth, sore throat, cough, hoarseness, and sometimes constipation or urinary discomfort; these are usually mild to moderate and often improve with continued use. Nasal dryness or nosebleeds and headache may occur in some patients, particularly with long-term use.
Serious or rare adverse effects
Serious but less common effects include worsening narrow-angle glaucoma (eye pain, blurred vision, seeing halos, red eyes), difficulty urinating or painful urination (especially in men with prostate enlargement or bladder outflow obstruction), fast or irregular heartbeat, severe allergic reactions (rash, swelling of face or throat, trouble breathing), and paradoxical bronchospasm with sudden worsening of wheezing or shortness of breath after dosing; these require immediate medical attention and stopping the drug until evaluated.
Warnings and precautions
Caution is advised in patients with narrow-angle glaucoma, urinary retention, enlarged prostate, bladder obstruction, or certain heart rhythm problems; they should be monitored for worsening symptoms. Safety in pregnancy is not fully established, so use is typically considered only if potential benefits outweigh risks; breastfeeding data in humans are limited, but systemic absorption from inhaled doses is low. The HandiHaler is approved for adult COPD only, while Respimat products have specific age indications that should be followed; use in very young children is not established.
Comparative safety
Overall, tiotropium is considered well tolerated for long-term maintenance compared with many systemic therapies, with fewer whole-body side effects than oral anticholinergics or frequent oral steroids, though it shares class-related anticholinergic risks and must be used correctly to minimize local throat irritation or bronchospasm.
Side-effect reporting and safety updates
Patients in the U.S. can report suspected side effects to FDA MedWatch (online or by phone) and should review the Medication Guide or patient leaflet that comes with their specific product for the most up-to-date safety information, including any new warnings.
Interactions and Precautions
Drug and product interactions
Using tiotropium with other anticholinergic medicines (such as ipratropium, some bladder control drugs, certain antihistamines, or older antidepressants with strong anticholinergic effects) can increase side effects like dry mouth, constipation, blurred vision, and urinary retention. No major interactions with common asthma or COPD controllers (inhaled corticosteroids, long-acting beta agonists, leukotriene modifiers) are expected and they are often used together. Alcohol and food do not significantly affect inhaled tiotropium, but alcohol may worsen dizziness or dry mouth in some people.
Other precautions and conditions
People with narrow-angle glaucoma, urinary retention, enlarged prostate, bladder outflow obstruction, or significant heart rhythm disorders should use tiotropium cautiously and under close medical supervision. Patients with severe kidney impairment may have higher drug exposure and should be monitored more closely for anticholinergic side effects. It should not be used as the sole therapy in patients with rapidly worsening or life-threatening asthma or COPD.
Diagnostic and procedural considerations
Tiotropium does not usually interfere with routine blood tests or imaging, but clinicians may review its use when planning surgery or procedures involving anesthesia due to potential additive effects with other anticholinergics.
Monitoring needs
Routine monitoring includes checking symptom control, rescue inhaler use, lung function (such as spirometry), and watching for signs of anticholinergic effects, glaucoma symptoms, or urinary problems; periodic reassessment ensures the dose and device remain appropriate as conditions change.
Common Questions and Answers
Q: Is tiotropium the same as a rescue inhaler like albuterol?
A: No, tiotropium is a long-acting maintenance inhaler taken once daily to help keep airways open over time, while albuterol and similar medicines work quickly for short-term relief during sudden breathing problems.
Q: How long does it take for tiotropium to start working?
A: Some people notice easier breathing within a few hours of the first dose, but the full benefit for day-to-day symptoms and flare-up prevention usually appears after several days to a few weeks of daily use.
Q: Can I use tiotropium if I only have asthma and not COPD?
A: Certain tiotropium inhaler products are approved as add-on maintenance treatment for asthma in patients 6 years and older whose asthma is not well controlled with inhaled corticosteroids, but it should be used under guidance from an asthma specialist or primary clinician.
Q: What should I do if my mouth is very dry while using tiotropium?
A: Mild dry mouth is common and may improve with sugar-free gum, lozenges, or frequent sips of water, but if dryness is severe or comes with trouble urinating, eye pain, or vision changes, you should contact your healthcare professional promptly.
Q: Can I stop tiotropium once I feel better?
A: Because tiotropium is a maintenance treatment, stopping it on your own can lead to worsening symptoms or more flare-ups, so any change in your regimen should be discussed with the clinician managing your asthma or COPD.
Better Treatment, Lower Cost – No Catch.
Find safer, more effective medications with fewer side effects – often for less money. It’s fast, free, and personalized. Learn More →
Disposal Guidance
Storage
Store tiotropium inhalers at room temperature away from excessive heat, cold, and moisture, and keep the device tightly closed or capped when not in use; keep out of reach of children and pets.
Product-specific notes
HandiHaler capsules should remain in the blister pack until immediately before use and should never be swallowed; Respimat cartridges should be stored in the device and used within the time frame specified in the patient instructions after first use.
Disposal
Discard the inhaler device and any remaining capsules or cartridges after the labeled number of doses or after the recommended in-use period; follow local guidelines for disposing of inhalers, and return unused or expired medications to a pharmacy take-back program if available rather than throwing them in household trash or wastewater.