At a Glance
How It Works
Brinsupri is a targeted medicine that calms overactive white blood cells in the lungs to reduce inflammation and bronchiectasis flare-ups.- It blocks an enzyme called dipeptidyl peptidase-1 (DPP1), which normally switches on powerful enzymes inside neutrophils (a type of white blood cell).
- By limiting activation of these enzymes, it helps reduce ongoing lung tissue damage and excessive mucus production.
- Over time, this can lower the number of lung exacerbations and help preserve lung function.
Treatment and Efficacy
Approved indications: Brinsupri is FDA-approved as a once-daily oral treatment for non-cystic fibrosis bronchiectasis in adults and adolescents 12 years of age and older.
Off-label and research uses: Because Brinsupri is newly approved and specifically labeled for non-cystic fibrosis bronchiectasis, there are currently no well-established off-label uses in routine practice; it is being studied for other neutrophil-driven inflammatory diseases, so use outside bronchiectasis is generally limited to clinical trials.
Efficacy expectations: In large clinical studies, Brinsupri added to standard bronchiectasis care reduced the annual rate of pulmonary exacerbations by about 20%, increased the chance of remaining exacerbation-free over a year, and at the 25 mg dose helped slow decline in lung function compared with placebo. Benefits build gradually over weeks to months, and many patients may not feel a dramatic daily change but experience fewer severe flare-ups, antibiotic courses, and hospitalizations over time.
Comparison with other treatments: Brinsupri is the first medicine approved to directly target the underlying neutrophil-related inflammation in bronchiectasis and is used alongside (not instead of) airway clearance techniques, inhaled therapies, and antibiotics; it offers a non-antibiotic mechanism distinct from chronic macrolides or inhaled antibiotics and can be an important option when exacerbations persist despite usual care.
Dosage and Administration
Typical dosing: For adults and adolescents 12 years and older with non-cystic fibrosis bronchiectasis, the usual Brinsupri dose is 10 mg or 25 mg taken by mouth once daily, with or without food, at about the same time each day.
How to take it: Take the tablet by mouth with water and try to take it consistently each day; check with your clinician before cutting, crushing, or chewing the tablet, and continue your other bronchiectasis treatments (such as airway clearance and inhaled medicines) unless your clinician advises changes.
Special dosing instructions: Your prescriber will choose between 10 mg and 25 mg based on how active your disease is, how you tolerate treatment, and other medical conditions; do not change the dose or stop Brinsupri on your own because its benefits depend on regular long-term use.
Missed dose and overdose: If you miss a dose, simply take your next dose at the usual time the following day and do not take extra tablets to make up for the missed one. In case of accidental overdose, contact your local poison control center or seek emergency medical care immediately, as there is no specific antidote and management focuses on monitoring and treating symptoms.
Safety and Side Effects
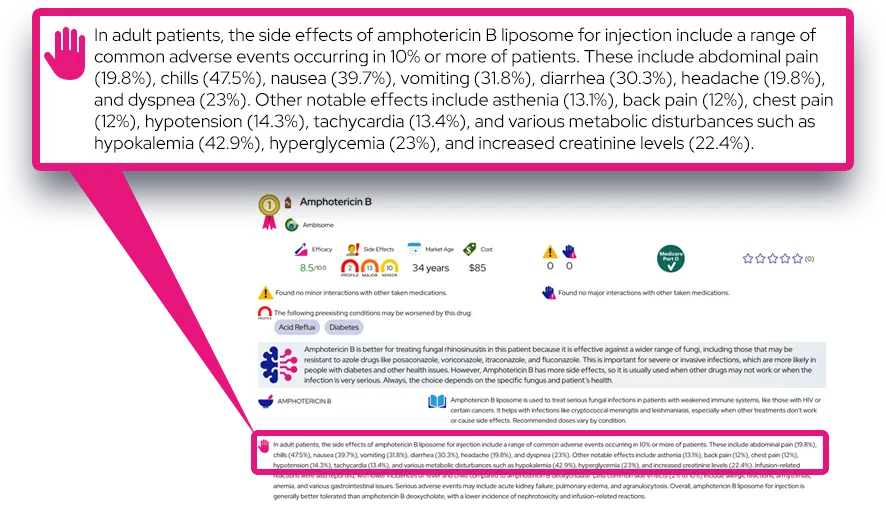
Common side effects: The most frequent side effects are upper respiratory tract infections, headache, rash, dry skin, small thickened skin patches (hyperkeratosis), and increased blood pressure; these are usually mild to moderate and often appear in the first weeks to months of treatment.
Less common but important effects: Some people develop abnormal liver blood tests, hair loss (alopecia), gum or dental problems, or certain types of skin cancer, so regular skin examinations and routine dental care are recommended while taking Brinsupri.
Serious reactions needing urgent care: Seek immediate medical attention for signs of a severe allergic reaction (such as trouble breathing, swelling of the face, lips, tongue, or throat, or widespread rash), rapidly changing or painful skin lesions, severe or unexplained gum bleeding or tooth pain, chest pain, sudden worsening shortness of breath, or symptoms of very high blood pressure such as severe headache or vision changes.
Warnings and precautions: There are no formal contraindications, but safety in pregnancy and breastfeeding has not been established, so use requires a careful risk–benefit discussion; Brinsupri is approved only for adults and adolescents 12 years and older, and its safety is unknown in younger children. No routine dose adjustment is needed in mild to severe kidney impairment down to an estimated GFR of about 15 mL/min/1.73 m² or in mild to severe liver impairment, but experience is limited in end-stage kidney disease and in dialysis patients.
Overall safety profile: In trials, Brinsupri was generally well tolerated compared with placebo and, unlike chronic antibiotics, does not promote antibiotic resistance, but it carries specific risks of dermatologic and dental side effects and possible skin cancers that require ongoing monitoring.
Side-effect reporting and updates: Report any side effects promptly to your prescriber; they can also be reported to the manufacturer’s safety line or to the FDA MedWatch program, and your healthcare team will stay informed about evolving safety communications and adjust treatment if needed.
Interactions and Precautions
Other prescription and OTC medicines: Brinsupri is broken down mainly by the liver enzyme CYP3A and is transported by P-glycoprotein, so strong or moderate CYP3A/P-gp inhibitors (for example some macrolide antibiotics like clarithromycin, certain azole antifungals, and some HIV or hepatitis antivirals, as well as verapamil) can increase Brinsupri levels, while strong CYP3A inducers (such as rifampin, carbamazepine, phenytoin, or certain HIV medicines) can lower its levels; your prescriber may avoid these combinations, adjust other drugs, or monitor you more closely. Brinsupri itself has limited effects on most common medicines, but because it can affect certain drug transporters, all prescription and over-the-counter drugs should be reviewed by your clinician or pharmacist.
Supplements, herbs, alcohol, and foods: Brinsupri can be taken with or without food, and there are no specific food restrictions identified; however, herbal supplements that strongly affect liver enzymes (such as St John’s wort) may alter Brinsupri levels and should not be used without medical advice. Moderate alcohol use has not been specifically studied with Brinsupri, but heavy drinking is discouraged because it can worsen overall health and blood pressure and complicate monitoring.
Vaccines and procedures: Because Brinsupri modifies activation of certain enzymes inside white blood cells, the safety of live attenuated vaccines during treatment is uncertain, and live vaccines are generally avoided while you are taking it; inactivated (non-live) vaccines are typically acceptable. There are no known specific interactions with X-rays, CT scans, MRI, or contrast dyes, but always tell surgical, anesthesia, and radiology teams that you are using Brinsupri.
Conditions needing extra caution and monitoring: Extra care is needed if you have a history of skin cancer, uncontrolled high blood pressure, significant dental or gum disease, or if you are pregnant or breastfeeding. Your clinician may recommend regular blood pressure checks, dermatologic and dental examinations, and sometimes periodic blood tests (including liver function) while you are on Brinsupri to detect problems early.
Common Questions and Answers
Q: How long does it take for Brinsupri to start working?
A: In clinical studies, benefits such as fewer bronchiectasis flare-ups emerged over weeks to months, so you should not expect an immediate change after the first few doses even though the medicine is already working in your lungs.
Q: Will Brinsupri replace my inhalers or airway clearance treatments?
A: No, Brinsupri is usually added to your existing bronchiectasis regimen and is meant to work alongside inhaled medicines, airway clearance techniques, and antibiotics rather than replace them.
Q: Is Brinsupri an antibiotic or a steroid?
A: Brinsupri is neither an antibiotic nor a steroid; it is a DPP1 inhibitor that acts inside certain white blood cells to reduce damaging inflammation that drives bronchiectasis.
Q: Can children take Brinsupri?
A: Brinsupri is approved for adults and adolescents 12 years of age and older, and its safety and effectiveness have not been established in children younger than 12.
Q: Do I need regular tests while taking Brinsupri?
A: Your clinician will typically monitor your blood pressure and check your skin, gums, and teeth, and may periodically order blood tests, and you should promptly report any new skin changes or dental symptoms.
Q: What should I do if I forget a dose of Brinsupri?
A: If you miss a dose, just take your next dose at the regular time the following day and do not take extra tablets to make up for the missed one.
Better Treatment, Lower Cost – No Catch.
Find safer, more effective medications with fewer side effects – often for less money. It’s fast, free, and personalized. Learn More →
Disposal Guidance
Storage: Store Brinsupri tablets at room temperature (68°F to 77°F / 20°C to 25°C) in the original bottle, tightly closed, away from moisture, heat, and direct light, and keep them out of the reach of children and pets.
Disposal: When tablets expire or are no longer needed, use a local medicine take-back program if available or ask your pharmacist how best to dispose of them; if no program exists, place tablets (preferably mixed with an undesirable substance and sealed in a container or bag) in household trash, and do not flush them down the toilet unless specifically instructed by local guidance.